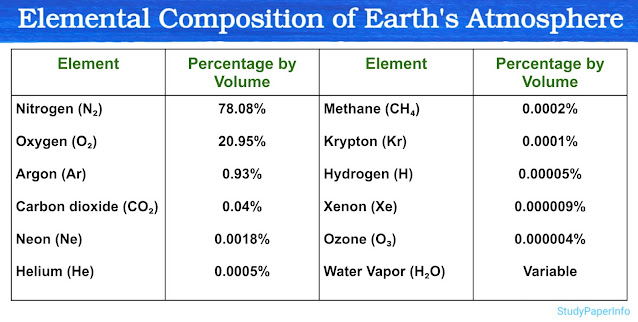
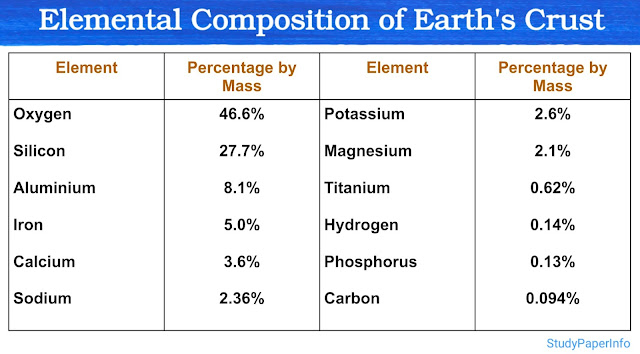
Subatomic Particles

Atoms are the basic units of matter and are made up of smaller components called subatomic particles. There are many types of subatomic particles known to science, but in the context of basic atomic structure, only three are considered most important: electrons, protons and neutrons. These three particles differ in their location, charge and mass. Together, electrons, protons and neutrons form the complete structure of atoms. Their arrangement and interaction define the atom's properties, chemical behavior, and participation in physical and chemical processes. These subatomic particles laid the foundation of modern atomic theory and quantum chemistry. 1. Electron Electrons are negatively charged subatomic particles. They are extremely small in mass and are found outside the nucleus of the atom in specific regions called orbitals or shells. The charge of an electron is −1 and its mass is approximately 1/1836 of a proton or neutron, which is around 9.1 × 10⁻³¹ kg, meaning it is n...