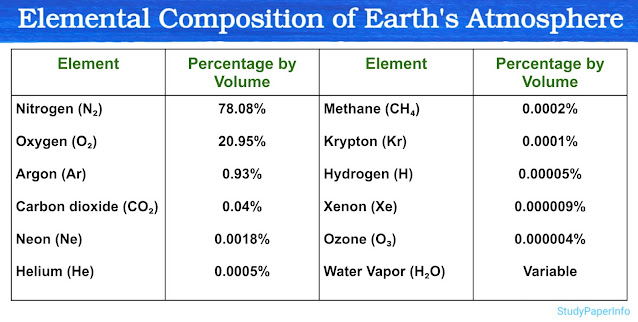
Describe Elemental Composition of Earth's Atmosphere
The Earth's atmosphere is a layer of gases that surrounds the planet and is held in place by gravity. This gaseous envelope plays a vital role in supporting life by providing oxygen for respiration, carbon dioxide for photosynthesis and protection from harmful solar radiation. It also regulates temperature, helps in weather formation and maintains pressure balance necessary for survival. The atmosphere is composed of a mixture of different elements and compounds, each present in specific proportions. Some of these gases are present in large quantities while others occur in very small traces. However, even those present in minute amounts can have significant environmental or biological roles. The composition of the atmosphere is generally stable up to around 80 to 100 kilometers from the Earth's surface and this region is known as the homosphere where gases are well mixed. The major components of this layer are nitrogen, oxygen and argon, while several other gases like carbon dioxide, neon, helium, methane and water vapor occur in smaller proportions. Understanding the exact elemental composition of the atmosphere is essential in environmental biology, climatology and global ecological studies.
The composition is measured by volume percentage and can be divided into three categories based on abundance: major components, minor components and trace gases.
1. Major Components
There are two major gases that dominate the Earth's atmosphere:
- Nitrogen (N₂)
- Nitrogen (N₂) is the most abundant gas, making up 78.08 percent of the atmosphere by volume. It is a chemically inert gas under normal conditions and plays a key role in the nitrogen cycle. Although it is not directly used in respiration, nitrogen is essential for the synthesis of amino acids, proteins, and nucleic acids in plants and animals.
- Oxygen (O₂)
- Oxygen (O₂) is the second most abundant gas, contributing 20.95 percent. It is crucial for aerobic respiration in almost all living organisms. Oxygen also supports combustion and forms ozone in the upper atmosphere which protects Earth from harmful ultraviolet radiation.
2. Minor Components
These gases are present in amounts less than 1 percent but still have important physical and biological roles:
- Argon (Ar)
- Argon (Ar) makes up 0.93 percent of the atmosphere. It is a noble gas, chemically inert and does not react with other elements. It is mainly used in industrial processes and does not play any direct role in biological systems.
- Carbon Dioxide (CO₂)
- Carbon Dioxide (CO₂) is present at 0.04 percent. Despite its low concentration, it plays a critical role in the greenhouse effect and climate regulation. It is also essential for photosynthesis in plants and is produced during respiration and combustion.
- Neon (Ne)
- Neon (Ne) is found at 0.0018 percent. It is another inert noble gas with no biological significance but is used in lighting and electronic applications.
- Helium (He)
- Helium (He) is present at 0.0005 percent. It is light, inert and non-reactive. It is mainly found in the upper atmosphere and is used in scientific and medical instruments.
3. Trace Gases
These are present in extremely small amounts, but some of them have strong environmental and climatic effects:
- Methane (CH₄)
- Methane (CH₄) is found at 0.0002 percent. It is a powerful greenhouse gas and is released from wetlands, livestock and fossil fuel extraction.
- Krypton (Kr)
- Krypton (Kr) is at 0.0001 percent. It is chemically inert and used in lighting and lasers.
- Hydrogen (H)
- Hydrogen (H) occurs at 0.00005 percent. Although it is the lightest element, its presence is very low in the atmosphere due to its tendency to escape Earth's gravity.
- Xenon (Xe)
- Xenon (Xe) is found at 0.000009 percent. It is an inert gas used in anesthesia and special lighting.
- Ozone (O₃)
- Ozone (O₃) is present at 0.000004 percent near the surface but is more concentrated in the stratosphere. It forms the ozone layer which absorbs harmful ultraviolet rays from the Sun.
- Water Vapor (H₂O)
- Water Vapor (H₂O) is highly variable in concentration depending on temperature and humidity. It can range from nearly zero in dry desert air to over 4 percent in hot and humid areas. It plays a crucial role in weather systems, precipitation and the greenhouse effect.

Comments
Post a Comment