Describe Chemical Element
A chemical element is a pure substance made up of only one type of atom, which is defined by its atomic number, i.e., the number of protons in the nucleus of each atom. This atomic number gives the element its identity and distinguishes it from all other elements. For example, carbon always has six protons, so its atomic number is 6.
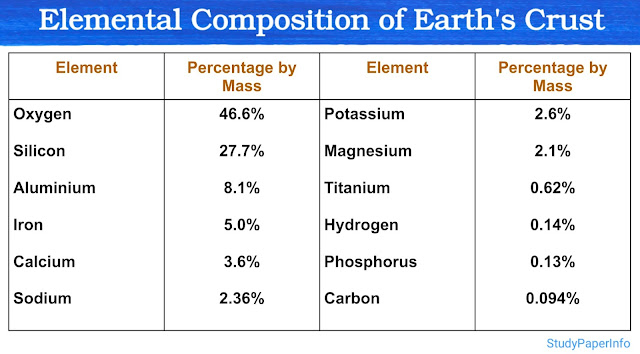
All substances found in nature, whether living or non-living, are made up of chemical elements or their compounds. These elements serve as the building blocks of all matter. For example, water is made from hydrogen and oxygen, rocks are made from silicon, oxygen, aluminum, etc., and living organisms are made mostly from carbon, hydrogen, oxygen, and nitrogen.
So far, 118 chemical elements have been discovered, out of which 94 occur naturally. The remaining are artificially synthesized in laboratories. Among these, 80 elements have stable isotopes. All elements with atomic numbers 1 to 82 have at least one stable isotope, except technetium (43) and promethium (61) which are radioactive. Elements with atomic numbers 83 and above like bismuth, uranium etc., are inherently unstable but some still occur in nature.
Out of all these elements, only about 15 elements are found in all living organisms, while 8 to 10 elements are present only in some specific organisms. Furthermore, more than 99% of atoms in animal bodies are made up of just four elements: hydrogen (H), oxygen (O), carbon (C) and nitrogen (N). These four are the primary components of water and organic molecules, which are essential for life.
Classification of Chemical Elements
Chemical elements can be classified in the following four major ways:
I. Classification Based on Atomic Form
II. Classification Based on Physical State
III. Classification Based on Metallic Character
IV. Classification Based on Biological Role
I. Classification Based on Atomic Form
Chemical elements may exist in nature either as single atoms or as groups of atoms bonded together. This gives rise to the classification into monoatomic and polyatomic elements.
This classification is important because the physical and chemical properties of these elements differ significantly depending on whether they exist as individual atoms or molecules.
1. Monoatomic Elements
These are elements whose atoms exist independently in the free state. Most of the noble gases (group 18 elements) such as helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe) are monoatomic in nature. These elements are chemically inert due to their complete outer electron shells and therefore they do not form molecules under normal conditions.
Some metals like copper (Cu), zinc (Zn), iron (Fe) also exist in metallic form as a lattice of individual atoms held together by metallic bonding. Even though they are not gases, they are considered monoatomic in the sense that each metallic atom in the solid contributes to the metallic structure independently.
2. Polyatomic Elements
These elements exist in molecular forms composed of more than one atom of the same element. Based on the number of atoms, these can be further divided into:
- Diatomic elements: Molecules made of two atoms. Examples include hydrogen (H₂), nitrogen (N₂), oxygen (O₂), fluorine (F₂), chlorine (Cl₂). These are commonly found in gaseous form.
- Polyatomic elements: Elements that naturally form molecules with more than two atoms. Examples include:
- Ozone (O₃): A triatomic molecule formed from oxygen.
- Phosphorus (P₄): White phosphorus exists as a tetra-atomic molecule.
- Sulfur (S₈): Exists in cyclic octatomic form in its most stable state.
II. Classification Based on Physical State
At standard room temperature and atmospheric pressure, elements exist in three physical states: solid, liquid and gas.
1. Solid Elements
The majority of elements exist in solid state under normal conditions. These elements usually have closely packed atoms with strong interatomic forces. These include:
- Metals like iron (Fe), copper (Cu), gold (Au), silver (Ag), aluminum (Al) etc. which are crystalline, shiny and malleable.
- Metalloids like silicon (Si), arsenic (As), antimony (Sb).
- Non-metals like carbon (C), phosphorus (P), sulfur (S), iodine (I) etc.
2. Liquid Elements
Only two elements are found in liquid state under standard conditions. This is a rare class and shows that phase state is not solely dependent on being metal or non-metal, but also on molecular interactions.
- Mercury (Hg): It is the only metal that is liquid at room temperature due to weak metallic bonding.
- Bromine (Br₂): A reddish-brown liquid non-metal with strong vapour pressure and corrosive nature.
3. Gaseous Elements
Gaseous elements have very weak intermolecular forces and high kinetic energy, hence they exist as vapour. There are eleven elements that exist as gases at room temperature. These include:
- Noble gases: He, Ne, Ar, Kr, Xe, Rn
- Diatomic non-metals: H₂, O₂, N₂, F₂, Cl₂
III. Classification Based on Metallic Character
Chemical elements are also classified based on their tendency to lose or gain electrons, which determines their metallic nature.
1. Metals
Metals are elements that lose electrons to form positive ions (cations). They are good conductors of heat and electricity. Metals have high melting and boiling points and are also malleable and ductile in nature. Examples: Sodium (Na), Calcium (Ca), Copper (Cu), Iron (Fe), Zinc (Zn)
Note: Metals occupy the left and center of the periodic table.
2. Non-Metals
Non-metals are elements that gain electrons to form negative ions (anions). They are poor conductors of heat and electricity, except graphite. In solid form, they are brittle and often exist as gases or soft solids.
Examples: Oxygen (O), Nitrogen (N), Sulfur (S), Phosphorus (P), Iodine (I)
Note: They are mainly found on the right side of the periodic table.
3. Metalloids (Semi-Metals)
These elements show intermediate properties between metals and non-metals. They are semiconductors and chemically versatile.
Examples: Boron (B), Silicon (Si), Germanium (Ge), Arsenic (As), Antimony (Sb), Tellurium (Te)
Note: They are placed along the zig-zag line between metals and non-metals in the periodic table.
IV. Classification Based on Biological Role
This is the most important classification from the biological and physiological point of view. Based on their role in living organisms, elements are grouped as:
1. Bulk or Major Elements
These are found in large amounts in all living organisms and are absolutely essential. These elements form over 99% of atoms in animal and plant cells. Hence, they are universal to life. These include:
- Hydrogen (H) and Oxygen (O): Together form water, the solvent of life. Also part of all organic molecules.
- Carbon (C): Backbone of all organic compounds like carbohydrates, proteins, fats, DNA, etc.
- Nitrogen (N): Important for amino acids, nucleotides, and proteins.
- Phosphorus (P): Involved in ATP, DNA, RNA and phospholipids.
- Sulfur (S): Present in amino acids (methionine, cysteine) and coenzymes.
2. Minor Elements (Essential Inorganic Ions)
These elements are needed in moderate amounts and are usually present in ionic forms. They represent about 0.5% of total body weight but are critical for physiological processes. Examples include:
- Sodium (Na⁺) and Potassium (K⁺): Maintain membrane potential and nerve impulse.
- Calcium (Ca²⁺): Essential for bone formation, muscle contraction, blood clotting.
- Magnesium (Mg²⁺): Cofactor in ATP reactions and enzymes.
- Chloride (Cl⁻): Maintains osmotic balance and acid-base regulation.
3. Trace Elements
These are required in very small amounts (micrograms or less), but play critical roles. Deficiency of these elements can result in serious health problems. Examples include:
- Iron (Fe): Central component of hemoglobin and cytochromes.
- Zinc (Zn): Involved in enzyme activity and immunity.
- Copper (Cu): Helps in iron metabolism and cellular respiration.
- Iodine (I): Required for synthesis of thyroid hormone.
- Cobalt (Co): Part of vitamin B₁₂, important in red blood cell formation.
- Selenium (Se): Antioxidant defense.
- Manganese (Mn): Bone health and enzymatic function.


Comments
Post a Comment